Data Clarification Form Template Clinical Trials

Whats new 2020 january 7 2020.
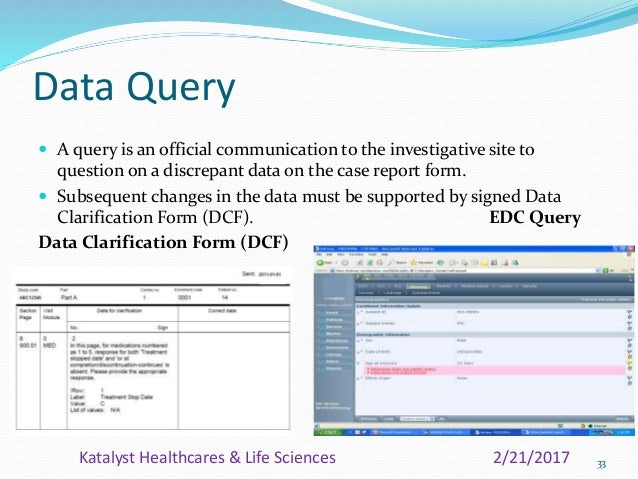
Data clarification form template clinical trials. Welcome to global health trials tools and templates library. Welcome to global health trials tools and templates library. A case report form crf is designed to collect the patient data in a clinical trial. A data clarification form dcf or data query form is a questionnaire specifically used in clinical researchthe dcf is the primary data clarification tool from the trial sponsor or contract research organization cro towards the investigator to clarify discrepancies and ask the investigator for clarification.
The templates below have been shared by other groups and are free to use and adapt for your research studies. Please ensure that you read and adapt them carefully for your own setting and that you reference global health trials and the global health network when you use them. Its development represents a significant part of the clinical trial and can affect study success site personnel capture the subjects data on the crf which is collected during their participation in a clinical trial. The nccih clinical research toolbox provides a web based information repository for investigators and staff involved in nccih funded clinical research.
The primary problems with crfs. This chapter includes the following topics related to creating and using data clarification forms dcfs. This template summarizes the data elements for submitting information about a statistical analysis for an outcome measure as described in the results data element definitions document and the final rule for. Please note that this page has been updated for 2015 following a quality check and review of the templates and many new ones have been added.
Samples forms and worksheets compliments of mountainside md press and conducting clinical research. The dcf is part of the data validation process in a clinical trial. 8 case report form the following example shows the type of information you will need to capture in a crf and a typical format for submission. Information on setting up dcfs is available in the discrepancy configuration chapter of the oracle clinical administrators guide.
The toolbox contains templates sample forms and information materials to assist clinical investigators in the development and conduct of high quality clinical research studies. Data clarification form template clinical trials posted on december 16 2019 december 19 2019 by admin simply as it is necessary for a fisherman to make use of the proper bait to draw that large fish its crucial that job seekers use the proper resume to draw that large job alternative. Preparing to apply for a u01 clinical trial registering with clinicaltrialsgov patient research registries clinical trial policies guidelines and templates.