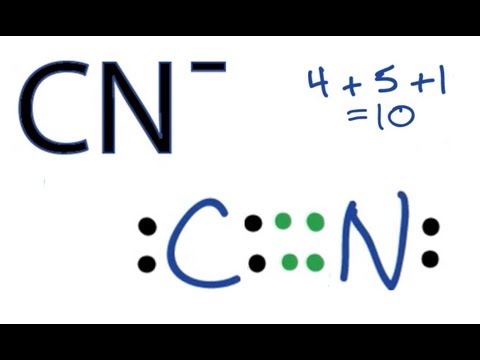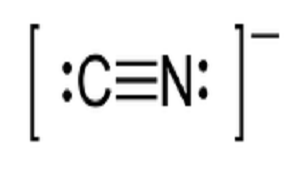Cn Lewis Diagram
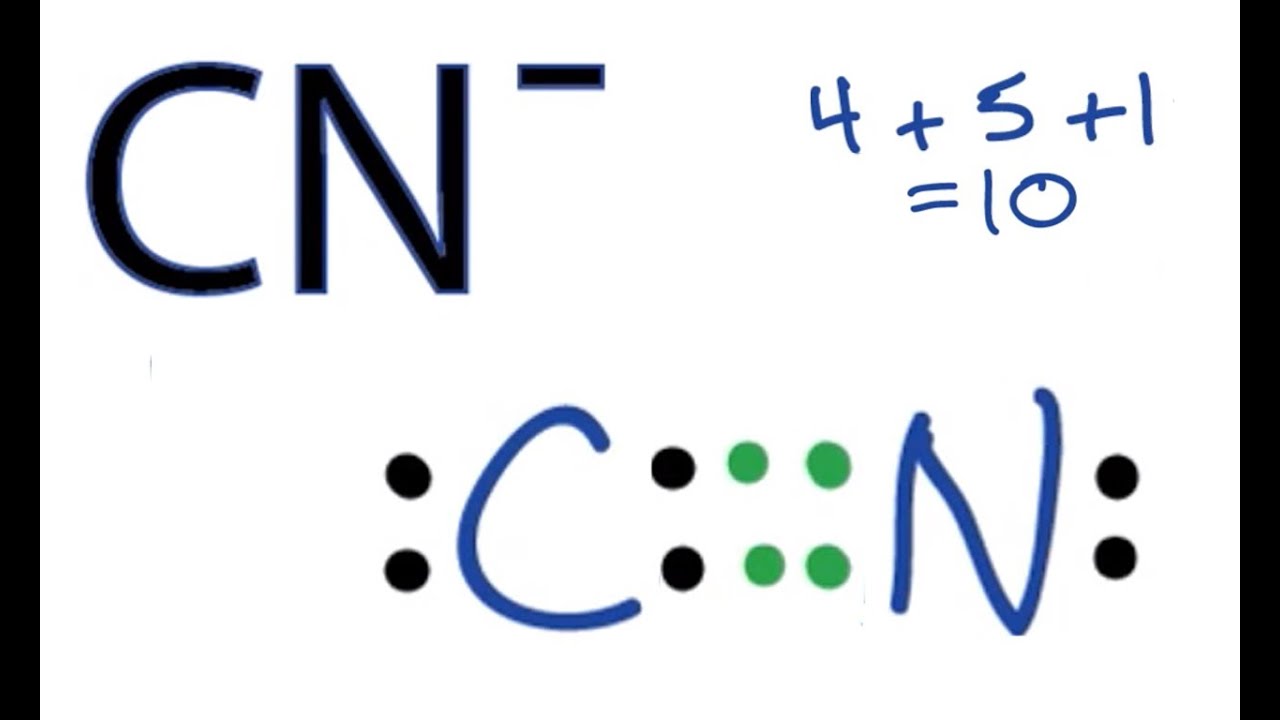
Many cyanide containing compounds are highly toxic but some are not.
Cn lewis diagram. For the cn lewis structure there are a total of 10 valence electrons available. A lewis structure also helps to make a prediction about the geometry of a molecule. Once we know how many valence electrons there are in clo we can distribute them around the central atom with the goal of filling the outer shells of each atom. A step by step explanation of how to draw the lewis structure for cn cyanide ion.
For the cn. The lewis structure for li is li with one dot to the right of the. Organic chemistry video lessons exam reviews acs video solutions solutions library. I also go over the hybridization shape and bond angle.
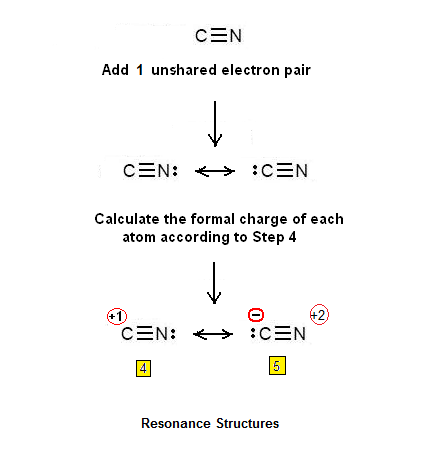
Consist of a carbon atom triple bonded to a nitrogen atom. It is highly poisonous. Lewis dot of cyanide ion. A video explanation of how to draw the lewis dot structure for the cyanide ion along with information about the compound including formal charges polarity hybrid orbitals shape and bond angles.
Draw lewis structure for cn cn and cn and. Watch the video solution for the question. In this video well write the correct name for cacn2 and then write the lewis structure for the compound. There arent enough valence electrons available for each atom to obtain an octet without sharing more than one pair.
70 more lewis dot structures. I quickly take you through how to draw the lewis structure of cn cyanideion. The lewis structure or lewis dot diagram shows the bonding between atoms of a molecule and any electrons that may exist. There should be brackets around the final lewis structure with a 1 charge on the outside.
Science chemistry video lessons exam reviews acs video solutions solutions library homework help. A lewis structure is a graphic representation of the electron distribution around atoms. C has 4 n has 5. Therefore cn has a triple bond between the carbon and oxygen atom.
Cn is a negative ion called an anion. The reason for learning to draw lewis structures is to predict the number and type of bonds that may be formed around an atom.