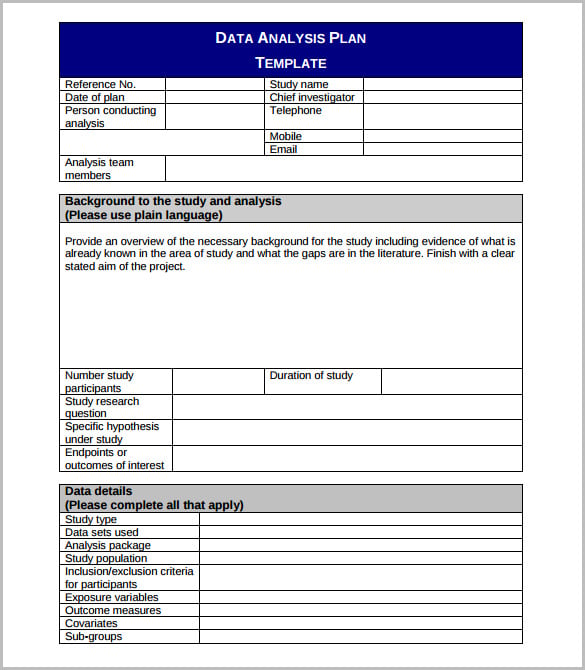
Research Data Collection Form Template

Investigator initiated clinical research data and safety monitoring guidelines and policies clinical study templates and forms nih and other federal guidelinespolicies for clinical research.
Research data collection form template. Read or download the 5 best data collection tools in 2019. To be used by biomedical and social behavioral investigators proposing secondary research with datawhich may include written text images or audio visual recordingsor biospecimens that were or will be collected for other purposes. Preparing to apply for a u01 clinical trial registering with clinicaltrialsgov patient research registries clinical trial policies guidelines and templates. The retired data collection forms webpage lists all prior versions arranged chronologically by the time frame used for data collection.
The best apps for gathering data in the field from our the ultimate guide to forms and surveys e book for free and start learning today. Forms and templates. Only research personnel will have access to the files and insert data collection and retention method and only those with an essential need to see names or other identifying information will have access to that particular file. Welcome to global health trials tools and templates library.
We have provided sample health worker data collection forms in a paper version for collecting data by hand and a spreadsheet version for collecting data on the computer. This log is not hard. The detailed template allows to record more information and can be used by six sigma practitioners. After the study is completed state time frame for retaining collect data and whether it will be destroyed.
Prior versions of the forms are available for review on the retired data collection forms webpage. Both require that you first specify the objectives of the data collection and identify the main study variables. Revise data collection forms to provide the minimum necessary dataset to answer policy and management questions. Data and data collection quantitative numbers tests counting measuring data collection techniques observations tests surveys document analysis the research literature quantitative methods key factors for high quality experimental design data should not be contaminated by poor measurement or errors in procedure.
Samples forms and worksheets compliments of mountainside md press and conducting clinical research. Please note that this page has been updated for 2015 following a quality check and review of the templates and many new ones have been added. Anonymous data collection consent form.