Eu Mdr Gap Analysis Template
Having no formal qa training i find myself creating a qas for the company i am working for.
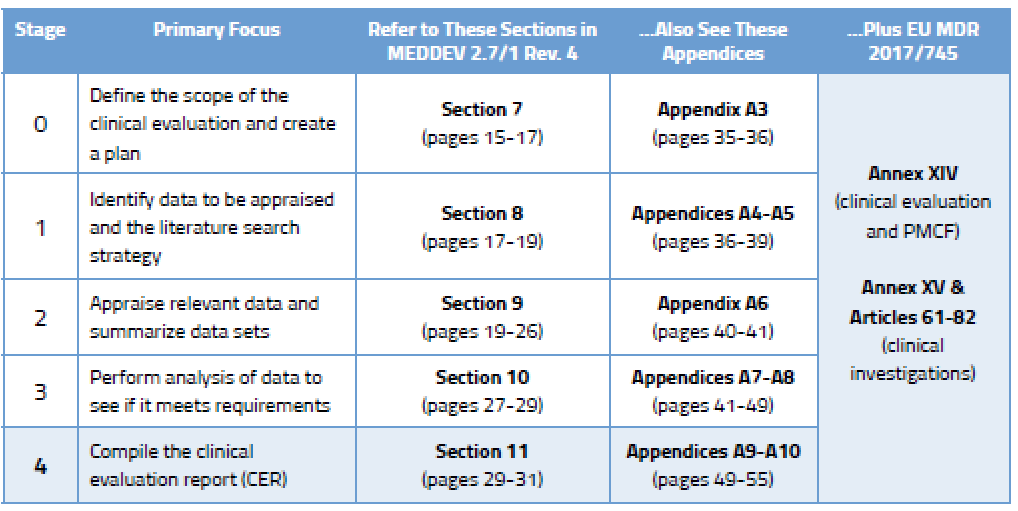
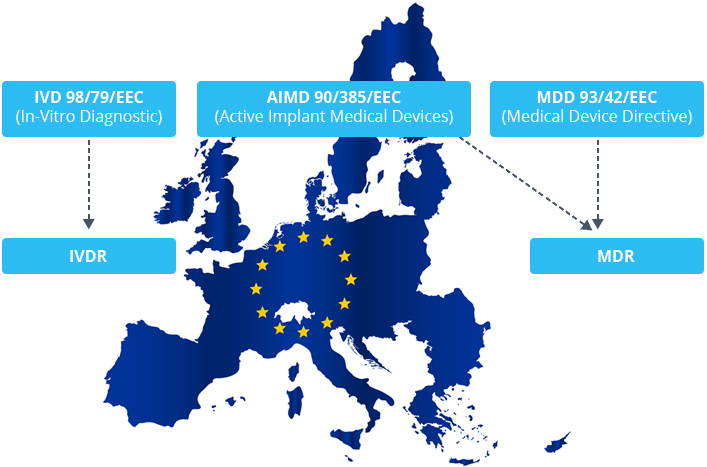
Eu mdr gap analysis template. I am trying to do a gap analysis to demonstrate to one of our customers that we comply to their required standard. Furthermore also a gap analysis of the new ivdr eu2017746 is available and we are also offer webinars and consulting. This new regulation whose full name is regulation eu 2017745 of the european parliament and of the council of 5 april. Mdr readiness review revision 1 july 2017 page 6 of 9 safety and performance requirements spr.
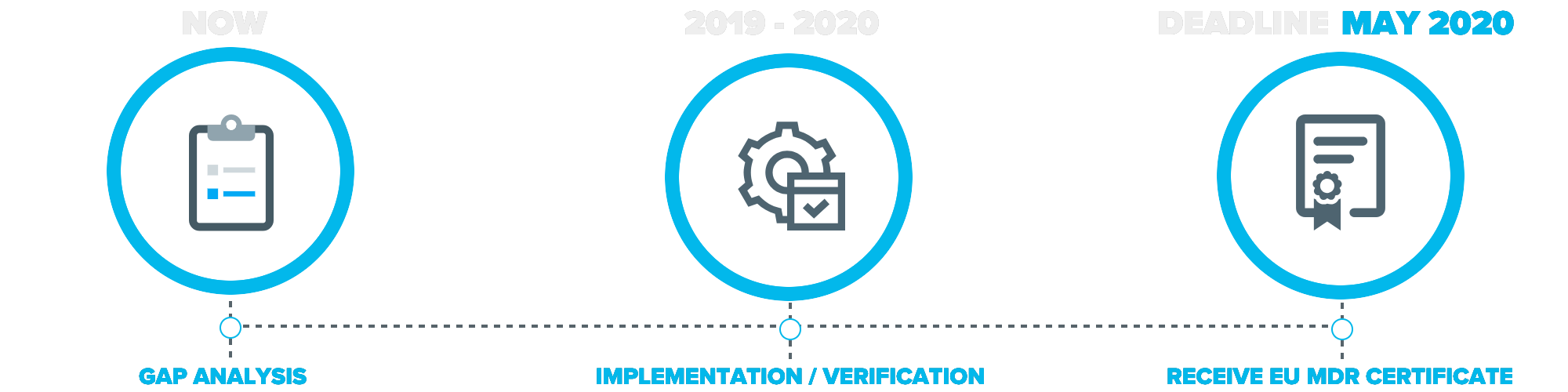
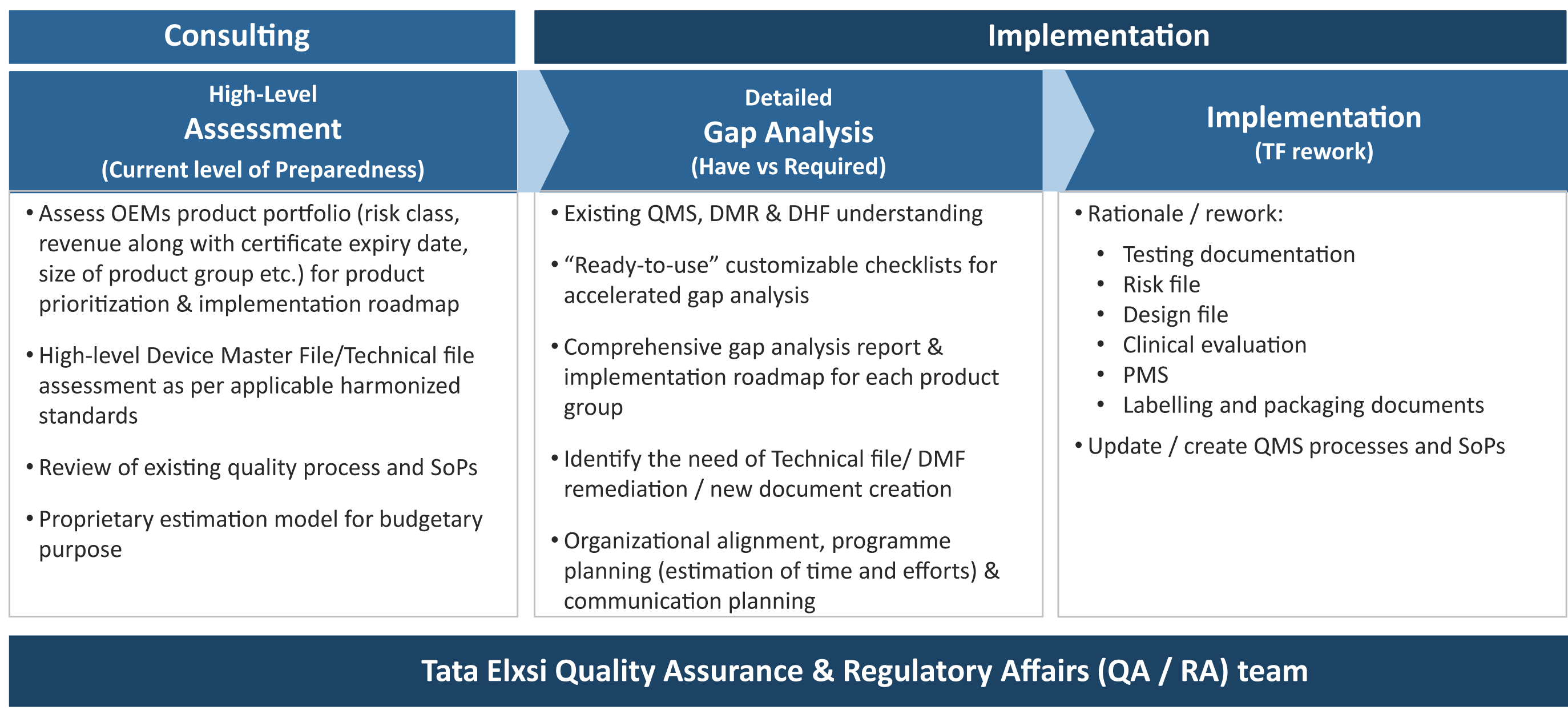
How to prepare for and implement the upcoming mdr dos and donts this has a more comprehensive review of each chapter in the mdr and what to pay attention to. Informational eu guidance on qualification and classification of software in regulation eu 2017745 mdr and regulation eu 2017746 ivdr. As mentioned in our recent blog a key first step is to conduct a thorough gap analysis to evaluate current capabilities and future requirements. Transitioning to the mdr might seem overwhelming and many companies dont know where to start.
We are a supplier for this customer and the analysis i am doing is on our vendors standards. These experts agreed that among the first steps a company should take is to conduct a gap analysis. On how to conduct parts of gap analysis while other sessions will address specific subjects of the mdr. Classification of non medical devicesaccessories as per new eu mdr classification rules.
A gap assessment provides a toolbox for the decision making process and should identify four key factors according to tony blank president of infinity biomedical group. Types of new test data necessary for mdr compliance. My question is this. This can be used as a gap analysis tool or as an aide memoire during your transition.
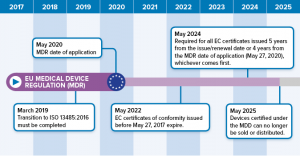
Mdr readiness review this is a nice sanity check for mdr readiness. The mdr gap analysis tool supports medical device companies to implement the new medical device regulation eu2017745 in a easy way. What i like about it is the. Greenlight guru has teamed up with eu mdr expert firm regulatory globe to offer a free mdr gap analysis tool to help companies with the transition process for compliance with new requirements for medical devices to be sold in the european market starting in 2020.
A thorough gap analysis will generate a task list for updating your procedures and documentation. European union medical device regulation mdr which was approved by the european parliament on april 5 2017 and was published in the of icial journal of the european union on 5th may 2017. Impact analysis and gap assessment. Devices that are also machinery within the meaning of point a of the second paragraph of article 2 of directive 200642ec of the european parliament and of the council 1 shall where a hazard relevant under that directive exists also meet the essential health and safety requirements set out in annex i to that directive to the extent to which those requirements are more specific than.
Eu mdd to mdr 2017745 transition strategy and plan. Scope of new data. The mdr tool can be downloaded in english or german language. This analysis should cover existing products as well as those in development estimating the costs and resources required to meet the requirements of the mdr or ivdr.
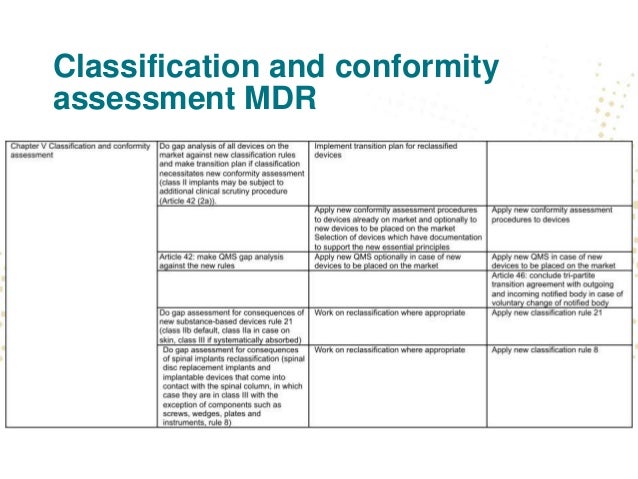
Guidance on qualification and classification of software in regulation eu 2017745 mdr and regulation eu 2017746 ivdr. Your first step should be to assess your current level of compliance. I was thinking to compare my documentation with annexes ii and iii of the mdr and i was wondering if any of you have a gap analysis. Classification and up classification of the devices as per new eu mdr classification rules.
Emergo can assist with this. List procedures records and examples that address the additional requirements. The roadmap to eu mdr implementation stay in compliance throughout the transition into.